Latest IR information
IR News
- 2026.02.17

- Announcement of FY2025 Grant Award for Research and Development of Orphan Drug for IV BCV for Adenovirus Infection Following Hematopoietic Stem Cell Transplantation


- 2026.02.17

- 株主通信「SymBio VISION vol.28」(Japanese only)


- 2026.02.09

- IV Brincidofovir Phase 2 Clinical Trial Results in CMV Infection Following Hematopoietic Stem Cell Transplantation Presented Orally at the Tandem Meetings


- 2026.02.05
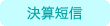
- Summary of Financial Statements for the Fiscal Year Ended December 31, 2025 [Japanese GAAP] (Consolidated)


- 2026.02.02

- Notice Regarding the Monthly Exercise Status of the 58th and 65th Stock Acquisition Rights (with Exercise Price Adjustment Clause) Issued through Third-Party Allotment


