SyB V-1901
antiviral drug
brincidofovir SyB V-1901: injection
overview
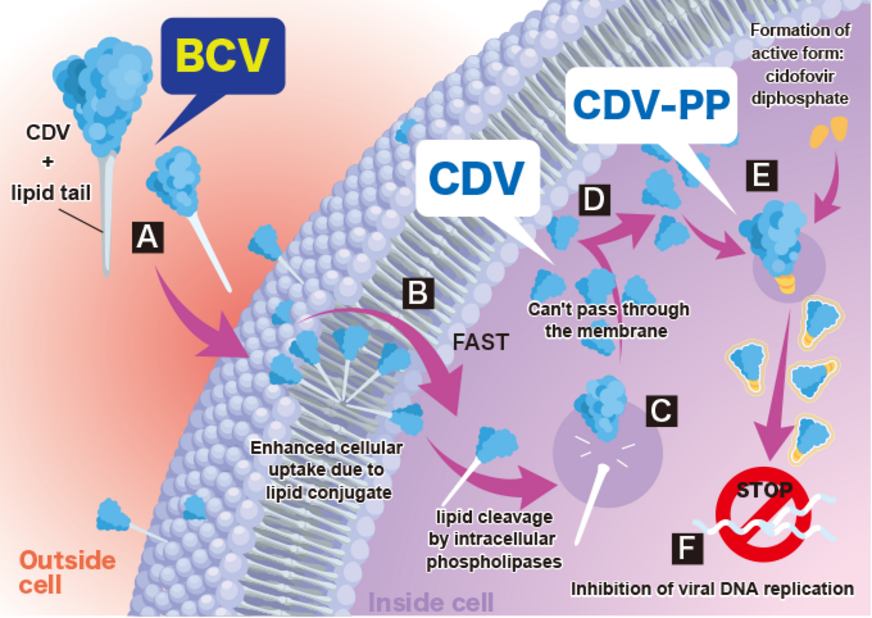
「SyB V-1901(注射剤)」(一般名:brincidofovir、BCV)は米国で承認されているシドフォビル(cidofovir:CDV、本邦は未承認)の脂質結合体で、CDVに比べて細胞内への取り込み効率が飛躍的に向上しています。BCVが細胞内に取り込まれるとCDV-PP(CDV diphosphate)に変換され、ウイルスの複製阻害作用により、より低用量で高い抗ウイルス効果を発揮します。また、BCVはCDVや他の抗ウイルス薬と比べ、広範囲の抗ウイルス効果を示します。すなわち、BCVはさまざまな2本鎖DNAウイルス感染症(サイトメガロウイルス、エプスタイン・バー ウイルス等のヘルペスウイルスや、アデノウイルス、BKウイルス、パピローマウイルス及び天然痘ウイルスやエムポックスウイルス等)に対して効果を示すと考えられています。さらに、CDVをはじめとする他の抗ウイルス薬に比べ、深刻な副作用である腎毒性や骨髄抑制を回避できるウイルス薬として期待されています。
このようなブリンシドフォビルの特性を活かし、シンバイオ製薬では移植後感染症、血液がん・固形がん、神経変性疾患の3つの治療領域に対して開発を進めています。
○移植後感染症領域
アデノウイルス
造血幹細胞移植後や臓器移植後などの免疫不全状態にある患者のアデノウイルス(AdV)感染症の治療を対象に、IV BCVのグローバル開発を優先的に進めることを決定し、2021年3月に、主に小児対象(成人も含む)のアデノウイルス感染症を対象とする第Ⅱa相臨床試験を開始するため、米国食品医薬品局(FDA)に治験許可申請(Investigational New Drug(IND)Application)を行いました。本開発プログラムについては、2021年4月に、FDAからファストトラック指定を受けています。2023年5月、本試験において、IV BCVの有効性を認め、ヒトPOC(Proof of Concept)が確立し、2024年上半期に、第Ⅱa相臨床試験は完了しました。現在、関係各国の規制当局との間で国際第Ⅲ相臨床試験の開始に向けて協議中で、同時に、当社では体制の構築を進めています。
サイトメガロウイルス
造血幹細胞移植後のサイトメガロウイルス感染症患者を対象とした米国における第Ⅱa相臨床試験は、2024年5月に開始し、同年6月に第1例目の登録が行われ、2025年4月末現在、症例登録数(累計)は、19例となっています。
ポリオーマウイルス
ポリオーマウイルス、特にJCウイルス(JCV)は、dsDNAウイルスの中でも、その感染によって脳に重篤な疾患を引き起こすことが知られており、既存の抗ウイルス薬ではほとんど効果が見られないため、有効な治療薬の開発が待ち望まれています。2022年11月に米国ペンシルベニア州立大学医学部との間で試料提供契約(MTA:Material Transfer Agreement)を締結し、ポリオーマウイルス感染マウスモデルにおけるBCVの抗ウイルス活性を検証する非臨床試験を実施しました。2024年7月には、その研究成果として、BCVの有用性に関する成績がmBio誌に公表されました。
○Hematology/Oncology Therapeutic Area
BCVは高い抗ウイルス活性に加え、抗腫瘍効果も確認されており、各国の研究機関との共同研究等を通じて、血液がん・固形がん領域における新規適応症の探索も行っています。現在、EBウイルス陽性リンパ腫に対するBCVの抗腫瘍効果とその機序の探索に関して、シンガポール国立がんセンターとの共同研究を実施しています。NK/T細胞リンパ腫・B細胞リンパ腫・末梢性T細胞リンパ腫(PTCL)等に対するBCVの抗腫瘍効果や、BCVの抗腫瘍効果を予測するバイオマーカーに関する共同研究成果は、2022年~2024年の間に、計5回、欧米の国際学会で発表されました。
2024年8月には、がん領域におけるIV BCVのFIH(First in Human)試験として、悪性リンパ腫患者を対象とした国際共同第Ⅰb相臨床試験を日本で開始し、現在はシンガポール、香港でも本試験が進行中です。本試験はBCVのがん領域におけるPOCを確立することを目的としています。また、2023年4月に、米国国立衛生研究所に所属する国立アレルギー・感染症研究所(NIAID :National Institute of Allergy and Infectious Diseases)との間で、EBウイルス関連リンパ増殖性疾患に対するBCVの有効性を評価する共同研究開発契約(CRADA)を締結しました。
○脳神経変性疾患
EBウイルスと多発性硬化症
全世界で約300万人が罹患していると推定されている難病の多発性硬化症(MS:Multiple Sclerosis)に関して、2022年1月にEBウイルス(EBV)感染がMSの主要発症因子であり、抗ウイルス薬によりEBVを標的とした治療の可能性がScienceで報告されました。またEBV感染とMSの発症機序に関する報告がNatureで相次いで報告されました。
シンバイオ製薬は2022年8月に、米国国立衛生研究所(NIH:National Institutes of Health)に所属する国立神経疾患・脳卒中研究所(NINDS:National Institute of Neurological Disorders and Stroke)との間で、共同研究試料提供契約(Collaboration Agreement for The Transfer of Human Materials)を締結し、2023年3月には、多発性硬化症の治療におけるBCVのEBVに対する効果を検証し、今後の臨床試験の実施に向けて必要とされる情報を得ることを目的として共同研究開発契約(CRADA:Cooperative Research and Development Agreement)を締結しました。2023年10月にはその研究成果が、第9回 ECTRIMS-ACTRIMS 合同学会(The 9th Joint ECTRIMS-ACTRIMS Meeting)において発表されました。現在、本共同研究では非ヒト霊長類であるマーモセットを用いた試験を実施しています。
単純ヘルペスウイルス1型とアルツハイマー型認知症
dsDNAウイルスの中には単純ヘルペスウイルス1型(HSV-1)をはじめ水痘帯状疱疹ウイルス(VZV)など、脳神経組織への指向性を有するものがあり、アルツハイマー型認知症(AD)を含めた様々な脳神経領域の疾患において、潜伏しているそれらウイルスの再活性化が関与している可能性を示す研究知見が増えています。2022年12月に米国タフツ大学により確立されたヒト神経幹細胞から構成される脳組織の三次元的模倣モデルにおいて、単純ヘルペスウイルス感染・再活性化によるAD様変化に対するBCVの効果を検証するための委託研究契約(Sponsored Research Agreement)を締結し、共同研究を実施しています。
共同研究について
現在、ウイルス感染症、血液がん・固形がん、神経変性疾患の各領域において、米国国立神経疾患・脳卒中研究所やフランスのグスタフ・ルーシー研究所など、世界中の著名な研究機関との間で共同研究開発を推進しており、共同研究成果の学会発表も積極的に行なわれております。詳しくは、下記ページをご参照ください。
ライセンス
2019年9月、シンバイオは、Chimerix, Inc.(キメリックス社、本社:米国ノースカロライナ州)との間で、BCVに関して、オルソポックスウイルス(天然痘やエムポックスなど)を除いたすべての疾患で世界全域対象の、開発・販売・製造を含めた独占的権利の取得を目的とするライセンス契約を締結しました。Smなお、2022年9月にキメリックス社からBCVのライセンスの譲渡を受けたEmergent BioSolutions Inc.の子会社であるEmergent Biodefense Operations Lansing LLCが、当社のライセンサーとなりました。
また、BCVはEU(欧州連合)における免疫不全患者におけるアデノウイルス感染症とサイトメガロウイルス感染症予防に対するオーファンドラッグ(希少疾病用医薬品)指定を受けています。